-
PDF
- Split View
-
Views
-
Cite
Cite
Anna Luerssen, Anett Gyurak, Ozlem Ayduk, Carter Wendelken, Silvia A. Bunge, Delay of gratification in childhood linked to cortical interactions with the nucleus accumbens, Social Cognitive and Affective Neuroscience, Volume 10, Issue 12, December 2015, Pages 1769–1776, https://doi.org/10.1093/scan/nsv068
Close - Share Icon Share
Abstract
Delay of gratification (DG) is the ability to forgo immediate temptations in the service of obtaining larger, delayed rewards. An extensive body of behavioral research has revealed that DG ability in childhood is associated with a host of important outcomes throughout development, and that attentional focus away from temptations underlies this ability. In this study, we conducted a functional magnetic resonance imaging study to identify the neural underpinnings of individual differences in DG among children. We observed a relationship between behavior during the classic DG task, a well-studied and ecologically valid measure, and functional connectivity during a modified version of this task in the scanner. Specifically, greater attentional focus away from temptations was associated with stronger functional coupling between the nucleus accumbens, a brain region that supports approach behavior, and several regions within prefrontal and parietal cortex that support self-control. These results shed light on the network interactions that contribute to DG and that account for individual differences in this capacity.
Introduction
Some children can wait until their homework is finished to watch television, whereas others have difficulty putting off playtime in favor of schoolwork. Some children effectively take turns, whereas others lash out when they do not get their way. Why? These differences partly reflect variations in delay of gratification (DG)—the ability to forgo an immediate reward in favor of receiving a more desirable reward later ( Mischel and Ayduk, 2011 ). Behavioral differences in this capacity have been studied extensively, and have been linked to important outcomes throughout development ( Mischel et al ., 1989 ).
In this study, we further assessed what distinguishes children during a DG challenge, focusing on neurobiology. More specifically, we measured the strength of communication between brain regions that support approach behavior and those that support self-control while children engaged in DG. Then, we evaluated whether variability in the connectivity of this network relates to individual differences in DG ability, as measured during the classic behavioral DG task, a well-studied and ecologically valid measure. This design addressed key limitations in the prior neuroimaging literature on DG, revealing the neural processes that support a child’s ability to delay gratification and providing insights into why and how children differ.
The Hot–Cool Framework of DG
DG is measured in childhood using the ‘Marshmallow’ or ‘Cookie’ task during which a child attempts to wait an extended period to obtain a larger treat rather than having a smaller treat that is immediately available (e.g. one cookie vs two; Shoda et al ., 1990 ). Behavior during this challenge in childhood (i.e. how long the child can wait for the larger treat) is linked to diverse indices of functioning in later life, including social adjustment, interpersonal aggression, drug use and even SAT scores (e.g. Mischel et al ., 1989 ; Ayduk et al ., 2000 ). As such, researchers have sought to understand what gives rise to DG ability. This research has served as the foundation for the Hot–Cool Framework, which argues that the capacity to successfully delay gratification is determined by the relationship between two interacting systems, the ‘hot-system’, associated with stimulus-control and the ‘cool-system’, associated with self-control ( Metcalfe and Mischel, 1999 ).
Which system is predominant is partly determined by attentional focus during the DG task. Spontaneously deploying attention (e.g. eye gaze) toward the rewards, which are the emotionally arousing, or ‘hot’ features of the situation, activates the hot-system to such a degree that the cool-system has difficulty overriding it ( Metcalfe and Mischel, 1999 ). In this way, the more ‘hot-focused’ a child is, the more they are impelled to eat the treats, and, therefore, the less time they are able to wait ( Rodriguez et al ., 1989 ; Peake et al ., 2002 ). In contrast, deploying attention away from the treats, to more benign or ‘cool’ features of the situation, allows the cool-system to down-regulate the hot-system and, therefore, helps the child to wait longer ( Rodriguez et al ., 1989 ; Metcalfe and Mischel, 1999 ; Peake et al ., 2002 ). This framework identifies attention deployment as a key mechanism underlying DG ability. In fact, attention deployment itself has been used by researchers to index DG beyond wait time, and has been linked to outcomes in later life, including inhibitory control ( Eigsti et al ., 2006 ).
Characterizing the hot- and cool-systems in the brain
The Hot-Cool Framework provides a conceptual foundation for exploring the neural basis of DG. Functional magnetic resonance imaging (fMRI) research with adults has built on this framework, showing that the ventral striatum (VS), particularly the nucleus accumbens (NAcc), part of a dopamine-related approach network, is a central player in the hot-system. For example, VS activation to rewards is stronger in adults who had difficulty with DG as children ( Casey et al ., 2011 ), and in those who more quickly devalue delayed rewards in delay discounting tasks ( Hariri et al ., 2006 ). Moreover, NAcc activation increases when adult participants choose a small, immediate option over a larger, delayed option ( McClure et al ., 2004 ), and relatedly, when smokers consider the immediate emotional pleasure of smoking rather than the long-term consequences ( Kober et al ., 2010 ).
This work additionally suggests that the dorsolateral prefrontal cortex (dlPFC) and lateral orbitofrontal cortex (lOFC), regions of the lateral prefrontal cortex (lPFC) linked to self-control (e.g. Stuss and Knight, 2013 ) are important for cool-system functioning. Activation in these lPFC regions increases when participants choose a large, delayed reward over a small, immediate one ( McClure et al ., 2004 ) and when smokers focus on the long-term costs of smoking, rather than the immediate pleasure ( Kober et al ., 2010 ).
In contrast with these lPFC regions, the medial (mOFC) appears to sit at the intersection between the hot- and cool-systems. On the one hand, the mOFC is associated with impulsivity during delay discounting tasks (e.g. McClure et al ., 2004 ). On the other hand, the mOFC determines the incentive value of stimuli given the larger context and regulates approach behavior (e.g. Arana et al ., 2003 ; Kringelbach and Rolls, 2004 ). Studies with non-human primates show that the NAcc receives input from the mOFC ( Ferry, et al ., 2000 ), and studies with humans have highlighted strong functional connections between the two ( Di Martino et al ., 2008 ). In fact, functional coupling between the mOFC and the NAcc is associated with behavioral persistence ( Jung et al ., 2010 ). The observation that medial regions of the cortex mature earlier than lateral regions ( Shaw et al ., 2008 ) suggests that the mOFC may be important for governing behavior in children, but in two plausible directions; by amplifying the bottom-up signals coming from the NAcc, or by regulating reward-related behavior in a top-down way.
While this neuroscientific research provides clues regarding the neural substrates of DG in children, the populations tested and measures employed in these studies are quite different from those associated with the classic DG research. For example, delay discounting tasks measure preferences for large, delayed rewards, whereas the classic task measures time and attention irrespective of preference (all participants who move forward with the task prefer the large, delayed reward). The classic task is also unique in that it requires participants to sustain their preference while the visible rewards serve as a constant temptation (e.g. Reynolds and Schiffbauer, 2005 ). This nicely parallels real-life situations and speaks to the task’s ecological validity. Although Casey et al . (2011) used childhood DG scores to predict brain activation during a response inhibition task, the participants were adults at the time of scanning. The fact that lPFC regions are among the last to mature ( Shaw et al ., 2008 ) begs the question of whether children’s cool-system is similar to adults’.
Finally, the latter neuroscientific studies largely evaluated differences in the magnitude of activation in distinct brain regions across conditions. Although these contrasts have been instrumental in identifying candidate loci of the hot- and cool-systems, they provide limited information about how these systems interact and the way these interactions relate to DG, a key focus of the Hot–Cool Framework. The aforementioned research on smokers took a step in addressing this issue by showing that increases in dlPFC activity are associated with decreases in VS activity when participants focus on the long-term consequences of smoking ( Kober et al ., 2010 ). A more recent study also found that participants who prioritized larger long-term rewards in a delayed discounting task exhibited stronger connectivity between the VS and the lPFC ( van den Bos et al ., 2014 ). These findings indicate that the strength of interactions between the VS and lPFC varies across individuals in ways that meaningfully relate to behavior.
The current research
In this study, we conducted functional connectivity analysis on fMRI data collected while participants tried to delay gratification. This analysis measures low-frequency fluctuations in the blood-oxygen-level-dependent (BOLD) signal and can be used to quantify the degree to which pairs of regions co-activate (e.g. Fox and Raichle, 2007 ). Prior research has found that the strength of coupling between brain regions varies across people and relates to individual differences, such as IQ ( Langeslag et al ., 2012 ) and reading skills ( Koyama et al ., 2011 ) in children. As such, functional connectivity analysis is appropriate for evaluating interactions between the hypothesized hot- and cool-systems and their relationship to DG ability.
Functional connectivity between brain regions can be measured under various conditions. In a standard resting-state scan, participants simply lie still in the scanner and are told not to engage in any specific task. Connectivity assessed during rest is thought to reflect the prior history of co-activation of brain regions over time (e.g. Cole et al ., 2014 ; Guerra-Carrillo et al . 2014 ). Functional connectivity can also be measured while participants actively complete a task (e.g. motor, gambling, emotion judgments, etc.). Research suggests that major brain networks are reliably detected across conditions, including both at rest and during tasks (e.g. Cole et al ., 2014 ), but that the strength of these connections can be modulated by task demands (e.g. Harrison et al ., 2008 ) and experience ( Guerra-Carrillo et al ., 2014 ).
Here, we assessed functional connectivity in a modified resting-state scan, under conditions designed to amplify DG. As with resting-state, children in our study were asked to lie still in the scanner. As in the classic DG task, however, children were given cookies that were placed on their chest and were told that they could either have one right away or two after the scan (see Methods for more details). To assess interactions between the hot- and cool-systems, we evaluated whether the strength of connectivity between the NAcc and the dlPFC, lOFC and mOFC during this scan correlated with an independent, standard and highly validated measure of DG ability: participants’ attention deployment during the classic task (e.g. Rodriguez et al ., 1989 ; Eigsti et al ., 2006 ). This measure was assessed behaviorally, before the MRI procedures. While the test re-test reliability of the classic task is largely unstudied, the longitudinal predictive power of one-shot assessments of DG behavior during this task suggests that it measures something temporally stable ( Mischel et al ., 1989 ; Ayduk et al ., 2000 ). Evaluating the association between DG behavior measured during one time point and functional connectivity in the brain measured at a second time point is a conservative approach.
We hypothesized that children who were more hot-focused, directing attention toward the rewards during the behavioral task, would exhibit weaker functional connectivity between the NAcc and one or more of these prefrontal regions when trying to delay gratification in the scanner. Such a pattern would suggest that these children have difficulty bringing their cool-systems online to regulate hot-system activation during DG challenges.
Materials and methods
Participants
We recruited forty-eight 7- to 9-year-old children. The lower and upper bounds of this age range were determined by two competing demands, our interest in studying children at an age when their DG skills are still developing (e.g. Rodriguez et al ., 1989 ), and the need to acquire high-quality fMRI data from as many children as possible for the planned functional connectivity analyses which are particularly susceptible to motion artifacts ( Power et al ., 2012 ).
Of this initial sample, 38 participants returned for the imaging session and successfully completed the fMRI DG task. As described further below, five additional participants were excluded due to noisy data, leaving 33 participants in the final analyses. As presented in Table 1 , the full sample and the subsample that was included in the final fMRI analyses did not differ on key variables such as gender, age, hot-focus or performance on the Wechlser Abbreviated Scale of Intelligence (WASI; Wechsler, 1999 ). However, the final fMRI sample had longer wait times on the behavioral DG task ( M = 22.81 min, s.d. = 5.67) compared with those who were excluded, ( M = 14.57 min, s.d. = 9.51), t (18.68) = −3.11, P < 0.01. See Table 2 for zero-order correlations across all key variables.
Participants included and excluded from fMRI analyses
| . | Statistic . | Included in fMRI analyses . | Excluded from fMRI analyses . | ||
|---|---|---|---|---|---|
| N | 33 | 15 | |||
| Female | 18 | 6 | |||
| Mean | s.d. | Mean | s.d. | ||
| Age (years) | t = 0.79 | 7.9 | 0.71 | 8.07 | 0.68 |
| Wait time (min.) | t = −3.11** | 22.81 | 5.68 | 14.57 | 9.51 |
| Hot-focus | t = 1.21 | 0.20 | 0.13 | 0.26 | 0.17 |
| WASI | t = 0.89 | 58.97 | 9.50 | 61.33 | 5.88 |
| . | Statistic . | Included in fMRI analyses . | Excluded from fMRI analyses . | ||
|---|---|---|---|---|---|
| N | 33 | 15 | |||
| Female | 18 | 6 | |||
| Mean | s.d. | Mean | s.d. | ||
| Age (years) | t = 0.79 | 7.9 | 0.71 | 8.07 | 0.68 |
| Wait time (min.) | t = −3.11** | 22.81 | 5.68 | 14.57 | 9.51 |
| Hot-focus | t = 1.21 | 0.20 | 0.13 | 0.26 | 0.17 |
| WASI | t = 0.89 | 58.97 | 9.50 | 61.33 | 5.88 |
Note. WASI, Wechsler Abbreviated Scale for Intelligence—matrix reasoning scores (standardized, with a mean of 50).
†P < 0.10, * P < 0.05. ** P < 0.01.
Participants included and excluded from fMRI analyses
| . | Statistic . | Included in fMRI analyses . | Excluded from fMRI analyses . | ||
|---|---|---|---|---|---|
| N | 33 | 15 | |||
| Female | 18 | 6 | |||
| Mean | s.d. | Mean | s.d. | ||
| Age (years) | t = 0.79 | 7.9 | 0.71 | 8.07 | 0.68 |
| Wait time (min.) | t = −3.11** | 22.81 | 5.68 | 14.57 | 9.51 |
| Hot-focus | t = 1.21 | 0.20 | 0.13 | 0.26 | 0.17 |
| WASI | t = 0.89 | 58.97 | 9.50 | 61.33 | 5.88 |
| . | Statistic . | Included in fMRI analyses . | Excluded from fMRI analyses . | ||
|---|---|---|---|---|---|
| N | 33 | 15 | |||
| Female | 18 | 6 | |||
| Mean | s.d. | Mean | s.d. | ||
| Age (years) | t = 0.79 | 7.9 | 0.71 | 8.07 | 0.68 |
| Wait time (min.) | t = −3.11** | 22.81 | 5.68 | 14.57 | 9.51 |
| Hot-focus | t = 1.21 | 0.20 | 0.13 | 0.26 | 0.17 |
| WASI | t = 0.89 | 58.97 | 9.50 | 61.33 | 5.88 |
Note. WASI, Wechsler Abbreviated Scale for Intelligence—matrix reasoning scores (standardized, with a mean of 50).
†P < 0.10, * P < 0.05. ** P < 0.01.
Zero-order correlations between key study variables
| . | Hot-focus . | Wait time . | Age . | WASI . | Gender . |
|---|---|---|---|---|---|
| Hot-focus | — | −0.23 | −0.22 | −0.04 | 0.01 |
| Wait time | −0.43** | — | 0.02 | 0.24 | 0.16 |
| Age | −0.29* | 0.08 | — | −0.11 | 0.25 |
| WASI | −0.04 | 0.15 | −0.08 | — | −0.11 |
| Gender | 0.11 | −0.10 | 0.13 | −0.10 | — |
| . | Hot-focus . | Wait time . | Age . | WASI . | Gender . |
|---|---|---|---|---|---|
| Hot-focus | — | −0.23 | −0.22 | −0.04 | 0.01 |
| Wait time | −0.43** | — | 0.02 | 0.24 | 0.16 |
| Age | −0.29* | 0.08 | — | −0.11 | 0.25 |
| WASI | −0.04 | 0.15 | −0.08 | — | −0.11 |
| Gender | 0.11 | −0.10 | 0.13 | −0.10 | — |
Note. Values below the diagonal are correlations across the entire sample ( n = 48). Values above the diagonal are correlations in the sample that was included in the final analyses ( n = 33). WASI, Wechsler Abbreviated Scale for Intelligence - matrix reasoning scores (standardized, with a mean of 50). Gender was scored with boys = 1, girls = −1.
†P < 0.10, * P < 0.05, ** P < 0.01.
Zero-order correlations between key study variables
| . | Hot-focus . | Wait time . | Age . | WASI . | Gender . |
|---|---|---|---|---|---|
| Hot-focus | — | −0.23 | −0.22 | −0.04 | 0.01 |
| Wait time | −0.43** | — | 0.02 | 0.24 | 0.16 |
| Age | −0.29* | 0.08 | — | −0.11 | 0.25 |
| WASI | −0.04 | 0.15 | −0.08 | — | −0.11 |
| Gender | 0.11 | −0.10 | 0.13 | −0.10 | — |
| . | Hot-focus . | Wait time . | Age . | WASI . | Gender . |
|---|---|---|---|---|---|
| Hot-focus | — | −0.23 | −0.22 | −0.04 | 0.01 |
| Wait time | −0.43** | — | 0.02 | 0.24 | 0.16 |
| Age | −0.29* | 0.08 | — | −0.11 | 0.25 |
| WASI | −0.04 | 0.15 | −0.08 | — | −0.11 |
| Gender | 0.11 | −0.10 | 0.13 | −0.10 | — |
Note. Values below the diagonal are correlations across the entire sample ( n = 48). Values above the diagonal are correlations in the sample that was included in the final analyses ( n = 33). WASI, Wechsler Abbreviated Scale for Intelligence - matrix reasoning scores (standardized, with a mean of 50). Gender was scored with boys = 1, girls = −1.
†P < 0.10, * P < 0.05, ** P < 0.01.
Procedure
Overview
During the behavioral session, participants first completed mock scanning, then the classic DG task, and subsequently the WASI matrix reasoning scale and a series of measures that were part of a larger study. The imaging session was scheduled after the behavioral session, with the date based on scanner and participant availability ( M = 43 days, s.d. = 31). Although the period between sessions was as long as 158 days, again, longitudinal research on DG intimates that the classic task measures something temporally stable, across months and even years ( Mischel et al ., 1989 ; Ayduk et al ., 2000 ). Participants completed a structural MRI scan while watching a cartoon, followed by the fMRI version of the DG task. These scans were followed by additional tasks unrelated to this study—an emotional go-no-go, and a measure of reactivity to rewards and punishments. If time permitted, at the end of the session, participants completed a pure rest scan during which they simply viewed a cross-hair.
We submitted the fMRI data to functional connectivity analyses, measuring patterns of low-frequency fluctuations of BOLD activation across time within each of several regions of interest (ROIs) in the brain. The strength of functional connectivity between two regions was computed as the average correlation of these BOLD fluctuations across the two ROIs over time, with higher r -values representing stronger temporal coupling between them ( Fox and Raichle, 2007 ).
Behavioral DG task
Following standard procedures (e.g. Mischel and Ebbesen, 1970 ), participants were situated in a bare experimental room. There was a computer nearby and a divider that blocked participants from seeing the rest of the room. Participants first established preference for a larger reward (two cookies instead of one). Next, the experimenter said s/he had to go out of the room and explained that if the participant could wait without eating any of the cookies or leaving their seat until the experimenter came back, the participant could have the larger reward they said they wanted. If the participant did not want to wait any longer, they could ring a bell situated on the table in front of them, and the experimenter would return. If they rang the bell, however, they could only have the smaller reward.
Once these contingencies were explained, the experimenter placed the participants’ options on a plate in front of them (two cookies on one side and one cookie on the other) and left the room. The experimenter returned either after the full 25-min waiting period [selected for this age group based on the procedures of Rodriguez et al ., (1989) ], or as soon as the participant rang the bell, ate any of the cookies, or left their seat. Wait time was the amount of time the participant waited in seconds. We did not analyze this variable as there was a ceiling effect in the data.
Participants’ behavior during the DG task was videotaped and subsequently coded for attention deployment. Using previously established coding procedures ( Rodriguez et al ., 1989 ), three trained coders categorized participants’ visual eye gaze each second, indicating whether the participant was attending to the rewards, the bell or elsewhere. During training, these coders established inter-rater reliability with an experienced coder using a prior set of DG task videos (average inter-rater agreement: 90.5%). Additionally, all three coders scored the same 8.3% of the current videos, with an average inter-rater agreement of 92.7%. Based on prior research ( Eigsti et al ., 2006 ), hot-focus during the DG task was computed as the proportion of the total wait time that the participant spent attending to the rewards or the bell.
fMRI DG task
While situated in the scanner, participants were reminded of the cookie game they played during the prior session. The experimenter explained that they were now going to play a slightly different version. In this version, the experimenter was going to start a new scan, and if the participant could wait until the scan finished all by itself, they could have two cookies. If, however, they did not want to wait, they could press any button on the button-box that they held in their hand. If they pressed the button, the experimenter would stop the scan and they would be given one cookie. Their options were placed on a tray on their chest as they lay in the scanner. They were told that once the scan began they would see live video feed of the tray. In reality, participants viewed a static photo of the cookies, presented using E-Prime 1.2 (Psychology Software Tools). No participants voluntarily reported disbelief.
Once participants understood the task contingencies, the experimenter returned to the control room and began the scan. For the first three participants, the scan duration was set to a maximum of 8 min. This maximum time was increased to 10 min for the remainder of the sample given the need to exclude periods involving excessive head movement from fMRI data analyses (see the Motion section for more information). Of the final 33 participants, three terminated the fMRI DG task but were included in the analyses given that they still had at least 5 min of usable data.
fMRI data acquisition and analysis
Imaging data were collected on a 3-Tesla Siemens Trio scanner using a 32-channel head coil. High-resolution anatomical images were collected with a T1-weighted spin-echo sequence (TI = 450, TR = 2300 ms, TE = 2.98 ms, 1 × 1 × 1 mm voxels). fMRI data were collected with a gradient-echo EPI sequence (TR = 2000 ms, TE = 20 ms, 36 axial slices collected in interleaved order, 3 × 3 × 3 mm voxels, 0.6 mm inter-slice gap, flip angle = 80°). Three hundred functional volumes were collected, with three volumes at the beginning of the scan removed to account for magnetic field equilibration.
Preprocessing
fMRI data were preprocessed and analyzed using Statistical Parametric Mapping (SPM) version 8 (Wellcome Trust Centre for Neuroimaging). Standard preprocessing steps were performed including slice-time correction, realignment, coregistration and smoothing using a Gaussian kernel of FWHM 6 mm. Images were normalized to standard space using the MNI template.
A priori ROIs
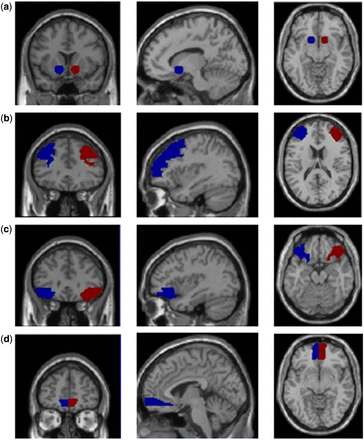
NAcc ROIs were created using MarsBar ( Brett et al ., 2002 ), as 8 mm spheres centered on MNI coordinates x = ± 14, y = 12, z = −8 ( Knutson et al ., 2007 ). Anatomical templates from the MNI database were used for the dlPFC, lOFC and mOFC ROIs ( Figure 1 ). We also used the MNI precentral gyrus (PCG) template in a discrimination analysis.
Key ROIs in the left and right hemispheres (blue and red clusters, respectively). Each row features a pair of homologous ROIs from coronal, sagittal and axial perspectives. (a) NAcc, (b) dlPFC, (c) lOFC, (d) mOFC.
Functional connectivity analysis
fMRI time series were extracted for each ROI using the following parameters. Mean and linear trends in the time series were removed. Six head motion parameters, corresponding to volume-to-volume translational and rotational movement, were regressed out, along with signal from the ventricles and white matter. Bandpass filters were applied to limit analyses to low-frequency signals (between 0.008 and 0.1 Hz) characteristic of resting-state functional connectivity (e.g. Fox and Raichle, 2007 ).
Motion
Given the pediatric sample and the sensitivity of functional connectivity analyses ( Power et al ., 2012 ), a conservative approach was adopted for eliminating movement confounds. In addition to regressing out motion in the time-series extraction, noisy volumes (greater than 1 mm movement) were identified with ArtRepair, a toolbox for SPM ( Mazaika et al ., 2009 ). These volumes were then removed using scrubbing procedures ( Power et al ., 2012 ). Participants were excluded from the analyses if more than 25% of their volumes were removed when scrubbing, and/or if they had <5 min of usable data remaining after scrubbing ( n = 5 removed). For participants fulfilling these inclusion criteria, their remaining volumes were concatenated and the analyses were conducted on these volumes ( Supplementary Table S1 for the total volumes and corresponding time analyzed for each participant). Finally, the average motion across volumes was included as a covariate in the DG analyses.
Results
Behavioral analyses
In the final fMRI sample, average wait time was 22.81 min (s.d. = 5.67). Twenty-five participants waited the entire 25-min period, and eight terminated. Hot-focus ranged from 0.01 to 0.50 ( M = 0.20, s.d. = 0.13). In the fMRI sample, the correlation between total wait time and hot-focus was not significant, but was in the theoretically expected direction, r = −0.23, P = 0.20, with higher hot-focus related to shorter wait times. In the full sample of 48 participants, this correlation was significant, r = −0.43, P < 0.01.
Functional connectivity analyses
Group-level ROI analyses
First, we measured average strength of functional connectivity among the hot- and cool-systems across participants by correlating the NAcc time-series with the dlPFC, lOFC and mOFC time-series ( Supplementary Table S2 ). These correlations were largely marginal or non-significant, with the exception of a positive correlation between the left NAcc and the left lOFC, r = 0.50, P < 0.01.
Individual differences ROI analyses
Next, we tested whether individual differences in functional connectivity among these ROIs correlated with DG ability. To this end, we computed Fisher z -transformations on the time-series correlations (given that correlations are not normally distributed), and correlated these z -scores with participants’ hot-focus on the behavioral DG task. Of these 12 correlations, all were negative, six were statistically significant and two were marginally significant ( Table 3 ). The six significant correlations stayed significant after accounting for multiple comparisons using the false discovery rate. The marginal correlations, however, became non-significant ( Supplementary Table S3 for corrected and uncorrected probabilities). Moreover, the same pattern of results was obtained when age, gender and WASI matrix reasoning standard scores ( Wechsler, 1999 ) were included as additional covariates. While not a planned analysis, we note that hot-focus also negatively correlated with the degree of functional connectivity between the left and right NAcc, r = −0.51, P < 0.01.
Hot-focus correlated with fronto-striatal functional connectivity
| . | Right dlPFC . | Left dlPFC . | Right lOFC . | Left lOFC . | Right mOFC . | Left mOFC . |
|---|---|---|---|---|---|---|
| Left NAcc | −0.09 | −0.30† | −0.45** | −0.30 † | −0.42* | −0.46** |
| Right Nacc | −0.03 | −0.14 | −0.06 | −0.49** | −0.43* | −0.50** |
| . | Right dlPFC . | Left dlPFC . | Right lOFC . | Left lOFC . | Right mOFC . | Left mOFC . |
|---|---|---|---|---|---|---|
| Left NAcc | −0.09 | −0.30† | −0.45** | −0.30 † | −0.42* | −0.46** |
| Right Nacc | −0.03 | −0.14 | −0.06 | −0.49** | −0.43* | −0.50** |
Note. Average motion was included as a covariate.
†P < 0.10, * P < 0.05, ** P < 0.01.
Hot-focus correlated with fronto-striatal functional connectivity
| . | Right dlPFC . | Left dlPFC . | Right lOFC . | Left lOFC . | Right mOFC . | Left mOFC . |
|---|---|---|---|---|---|---|
| Left NAcc | −0.09 | −0.30† | −0.45** | −0.30 † | −0.42* | −0.46** |
| Right Nacc | −0.03 | −0.14 | −0.06 | −0.49** | −0.43* | −0.50** |
| . | Right dlPFC . | Left dlPFC . | Right lOFC . | Left lOFC . | Right mOFC . | Left mOFC . |
|---|---|---|---|---|---|---|
| Left NAcc | −0.09 | −0.30† | −0.45** | −0.30 † | −0.42* | −0.46** |
| Right Nacc | −0.03 | −0.14 | −0.06 | −0.49** | −0.43* | −0.50** |
Note. Average motion was included as a covariate.
†P < 0.10, * P < 0.05, ** P < 0.01.
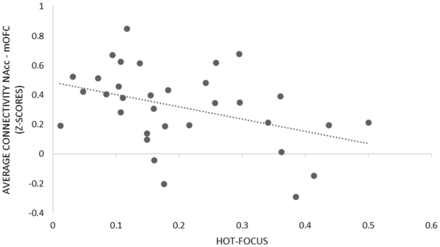
These results indicate that the more hot-focused a child was on the behavioral DG task, the weaker the functional connectivity between the candidate hot- and cool-systems while the children attempted to delay gratification in the MRI scanner. Figure 2 features a graph of hot-focus plotted against average ( z -scored) functional connectivity, in this case between the left and right NAcc and left and right mOFC.
Hot-focus, as measured during the behavioral DG task, plotted against average connectivity ( z -scores)—across left and right NAcc and left and right mOFC.
Discrimination analyses
It is possible, however, that there is nothing special about the set of regions we evaluated above, but rather that hot-focus is related to functional connectivity throughout the brain. To evaluate this alternative hypothesis, we calculated functional connectivity between the NAcc and a region within frontal cortex that is not closely linked to self-control—the PCG. At the group level, average functional connections between the NAcc and PCG were non-significant, (| r ’s| < 0.16, P ’s > 0.37). We then correlated these indices with participants’ hot-focus scores. None of these correlations was significant (| r ’s| < 0.13, P ’s > 0.48), indicating that the results above do not generalize across the frontal cortex.
We also evaluated the possibility that hot-focus relates to functional connectivity within the PFC (connectivity among the left/right dlPFC, lOFC and mOFC). At the group level, many of the average functional connections across PFC regions were significant ( Supplementary Table S4 ). As can be seen in Table 4 , none of the 15 functional connections correlated with hot-focus, however.
Hot-focus correlated with fronto-frontal functional connectivity
| . | Right dlPFC . | Left dlPFC . | Right lOFC . | Left lOFC . | Right mOFC . | Left mOFC . |
|---|---|---|---|---|---|---|
| Right dlPFC | — | −0.26 | 0.23 | 0.02 | 0.18 | 0.14 |
| Left dlPFC | — | — | 0.24 | −0.01 | 0.11 | 0.02 |
| Right lOFC | — | — | — | −0.07 | 0.02 | −0.07 |
| Left lOFC | — | — | — | — | −0.01 | −0.07 |
| Right mOFC | — | — | — | — | — | −0.03 |
| Left mOFC | — | — | — | — | — | — |
| . | Right dlPFC . | Left dlPFC . | Right lOFC . | Left lOFC . | Right mOFC . | Left mOFC . |
|---|---|---|---|---|---|---|
| Right dlPFC | — | −0.26 | 0.23 | 0.02 | 0.18 | 0.14 |
| Left dlPFC | — | — | 0.24 | −0.01 | 0.11 | 0.02 |
| Right lOFC | — | — | — | −0.07 | 0.02 | −0.07 |
| Left lOFC | — | — | — | — | −0.01 | −0.07 |
| Right mOFC | — | — | — | — | — | −0.03 |
| Left mOFC | — | — | — | — | — | — |
Note. Average motion was included as a covariate.
†P < 0.10, * P < 0.05, ** P < 0.01.
Hot-focus correlated with fronto-frontal functional connectivity
| . | Right dlPFC . | Left dlPFC . | Right lOFC . | Left lOFC . | Right mOFC . | Left mOFC . |
|---|---|---|---|---|---|---|
| Right dlPFC | — | −0.26 | 0.23 | 0.02 | 0.18 | 0.14 |
| Left dlPFC | — | — | 0.24 | −0.01 | 0.11 | 0.02 |
| Right lOFC | — | — | — | −0.07 | 0.02 | −0.07 |
| Left lOFC | — | — | — | — | −0.01 | −0.07 |
| Right mOFC | — | — | — | — | — | −0.03 |
| Left mOFC | — | — | — | — | — | — |
| . | Right dlPFC . | Left dlPFC . | Right lOFC . | Left lOFC . | Right mOFC . | Left mOFC . |
|---|---|---|---|---|---|---|
| Right dlPFC | — | −0.26 | 0.23 | 0.02 | 0.18 | 0.14 |
| Left dlPFC | — | — | 0.24 | −0.01 | 0.11 | 0.02 |
| Right lOFC | — | — | — | −0.07 | 0.02 | −0.07 |
| Left lOFC | — | — | — | — | −0.01 | −0.07 |
| Right mOFC | — | — | — | — | — | −0.03 |
| Left mOFC | — | — | — | — | — | — |
Note. Average motion was included as a covariate.
†P < 0.10, * P < 0.05, ** P < 0.01.
Exploratory whole-brain analyses
Although we primarily focused on regions of a priori interest, these were selected based on fMRI research involving adults and not children. Thus, we also conducted an exploratory whole-brain analysis to identify all voxels in the brain for which the strength of functional connectivity with the NAcc was correlated with hot-focus. We used a whole-brain mask to limit our analyses to brain tissue, and, again, included average head motion as a covariate. After conducting cluster-level correction, this analysis yielded a region in right lateral parietal cortex (140 voxels; peak at x = 66, y = −45, z = 36). See Supplementary Table S5 for additional clusters at a more liberal threshold.
Discussion
Attention deployment is a primary mechanism distinguishing children who are successfully able to delay gratification and those who have difficulties. In this study, we evaluated whether this attention deployment relates to individual differences in the functional connection between the NAcc, a brain structure that supports approach behavior, and prefrontal regions implicated in self-control. Indeed, planned analyses revealed that children who focused their attention toward the rewards during a behavioral DG task (i.e. higher hot-focus) exhibited weaker functional connectivity between the NAcc and the prefrontal regions examined, particularly the lOFC and the mOFC—while attempting to delay gratification in the MRI scanner. Exploratory whole-brain analyses revealed that the negative relationship between hot-focus and connectivity with the NAcc was even stronger in parietal cortex, a region known to interact closely with lPFC in the service of goal-directed behavior (e.g. Stuss and Knight, 2013 ).
Two of the significant connections (and two marginally significant connections) involved lPFC regions implicated in self-control. As previously described, these results were obtained in 7- to 9-year-olds despite evidence that these lPFC regions are both structurally and functionally immature at this age ( Shaw et al ., 2008 ). This may not be surprising given the contemporary view that while these regions are engaged less efficiently in children than in adults, they are not inactive (e.g. Anderson and Spencer-Smith, 2013 ). This suggests that there is still the potential for individual differences in lPFC functioning to emerge across children in this age range.
Although we cannot say that any of these prefrontal regions contributed more to individual differences in hot-focus than others, we note that the brain-behavior correlations were the most reliable for mOFC. Again, the mOFC has been associated with both impulsivity (e.g. McClure et al ., 2004 ) and behavioral regulation (e.g. Arana et al ., 2003 ; Kringelbach and Rolls, 2004 ; Jung et al ., 2010 ) in adults. Our results suggest that the mOFC may be engaged in a regulatory manner during DG challenges in childhood. Perhaps this region helps determine the incentive value of the two options, enabling prioritization of the large, delayed reward over the small, immediate one.
Collectively, these results fit nicely with research demonstrating that cortical–striatal connections are widespread and are essential to reward processing (e.g. Haber and Knutson, 2010 for review). We note, however, that while functional connectivity often reflects anatomical connections, it can also be detected between regions that are connected polysynaptically ( Buckner et al ., 2013 ). This is important to consider in making sense of the functional relationship between the NAcc and parietal cortex, as there are no known direct projections between these regions. We are not the first, in fact, to detect NAcc-parietal functional connectivity ( Cauda et al ., 2011 ).
We did not find that functional connectivity among prefrontal regions relates to DG behavior. This result stands in contrast to a recent study involving 6- to 13-year-olds showing that stronger ventromedial PFC–dlPFC connectivity correlates with reduced discounting of delayed rewards and with age ( Steinbeis et al ., 2014 ). This discrepancy may be due to the fact that participants in the latter study completed a delay discounting task rather than the classic DG task. Moreover, the broader age range (6–12 years, as opposed to 7–9) was perhaps better suited to discovering age-related changes in functional connectivity and behavior. Taken together, these two studies indicate that self-control in childhood is related to both long-range, fronto-striatal, and short-range, fronto-frontal, connectivity.
Implications and future directions
That we were able to identify individual differences in brain connectivity associated with DG is notable in light of the fact that a certain degree of self-control was necessary for successful fMRI data collection. Indeed, children who were unable to keep still during scanning had to be excluded from the analyses, and our final fMRI sample had longer wait times on the behavioral DG task than participants who were excluded (with 25 of the final 33 participants waiting the entire 25 min). That we detected a significant relationship between hot-focus and functional connectivity in this restricted sample may suggest that the true relationship is even stronger. It is also possible, however, that the most impulsive children, who could not meet the self-control requirements of the scanning environment, display a distinct pattern of functional connectivity when delaying gratification.
Here, we sought to identify differences in brain network connectivity during DG that could explain why some children exhibit better self-control than others. Thus, we adapted the standard resting-state fMRI paradigm to amplify DG demands. To determine whether the individual variability in network connectivity reported here reflects state or trait differences between children, future research should assess whether these results generalize to pure resting-state conditions. As a first step toward this goal, we collected pilot resting-state fMRI data from a subset of the children in this study. None of these correlations reached significance ( Supplementary Table S6 ). However, these results are inconclusive as we had usable resting-state data for only 15 of the children. Thus, it is still an open question as to whether children good at DG exhibit stronger fronto-striatal connectivity specifically when tempted by the possibility of an immediate reward or whether these reflect stable individual differences.
DG skills that are honed in childhood can be relied on throughout life, including adolescence when academic stress heightens at the same time as the draw to use drugs and alcohol. Thus, examining these processes in childhood is essential. Our results suggest that children whose attention is grabbed by environmental temptations may be less successful at bringing the cool-system online to down-regulate hot-system activation. We also know, however, that functional connectivity changes with experience ( Guerra-Carrillo et al ., 2014 ). Thus, practice with DG may reinforce connectivity in this fronto-striatal network. This topic is ripe for further research—research that is important to conduct given that DG ability is linked to a variety of favorable life outcomes.
Funding
This work was supported by the National Institute of Mental Health [T32 MH020006]; National Institutes of Health (R01 NS057146 to S.A.B.); and Greater Good Science Center [to A.G. and A.L].
Supplementary data
Supplementary data are available at SCAN online
Conflict of interest . None declared.
References